A strategy to replace the high loss of cells, especially cardiomyocytes, in injury is to deliver engineered cardiac cells or tissues (graft) into the damaged host tissue. Embryonic stem cells (ESCs) and Human-induced Pluripotent Stem Cells (hiPSC) are the most readily available sources of human-lineage to derive cardiomyocytes, because they can proliferate indefinitely and be differentiated into cells of different lineages. The key objective in cardiac tissue/cell engineering include: 1) reaching high proliferative efficacy, 2) high rate of cardiomyocyte differentiation, and 3) reaching efficient engraftment, also reducing host rejection to graft.
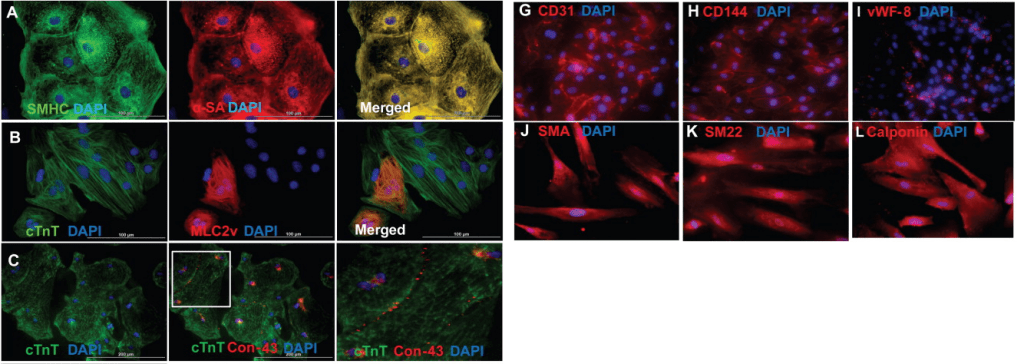
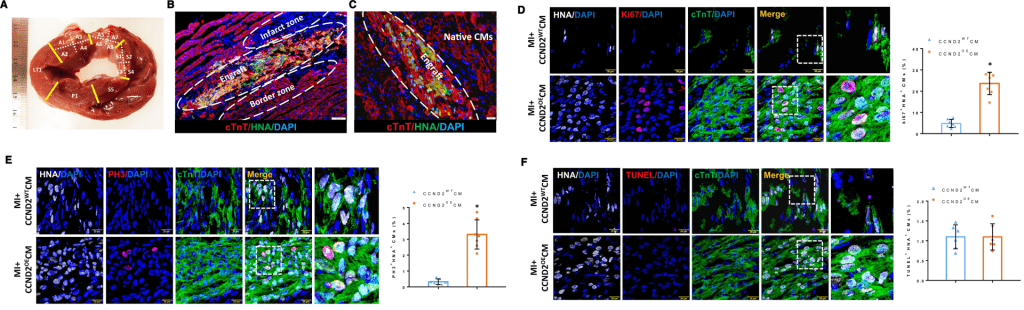
Zhang lab has mastered the technique to create hiPSC-derived cardiac cells for a long time. Thus, we focus on strategies to increase proliferate and engraftment rate of the engineered cells and tissues. In 2014, we, led by Dr. Ye Lei, created a new hiPSC-derived cardiac tissue, which consisted of cardiomyocytes, endothelial cells, and smooth muscle cells differentiated from the same hiPSC origin. This engineered tissue was successfully delivered into a porcine injured-heart model; the tissue survived in the host four weeks after delivery, demonstrated better engraftment rate and improve the host cardiac function compared to delivering hiPSC-derived cardiomyocytes alone. Then, in 2021, the work led by Dr. Meng Zhao showed that by overexpressing Cyclin D2 (CCND2), the hiPSC-derived cardiomyocytes not only significantly increased the engraftment rate but also promoted the host cardiomyocyte proliferation in the porcine model. Furthermore, exosomes, which contain microRNAs, released by CCND2-overexpressing hiPSC-derived cardiomyocytes interact with the host cardiomyocytes and support their proliferation.
Read more:
– Ye L, Zhang P, Duval S, Su L, Xiong Q, Zhang J. Thymosin β4 increases the Potency of Transplanted Mesenchymal Stem Cells for Myocardial Repair. Circulation. 2013;128(11 Suppl 1):S32-S41. PMID: 24030419; PMCID: PMC3886821
– Ye L, Chang YH, Xiong Q, Zhang P, Zhang L, Somasundaram P, Lepley M, Swingen C, Su L, Wendel JS, Guo J, Jang A, Rosenbush D, Greder L, Dutton JR, Zhang J, Kamp TJ, Kaufman DS, Ge Y, Zhang J. Cardiac Repair in a Porcine Model of Acute Myocardial Infarction with Human Induced Pluripotent Stem Cell-Derived Cardiovascular Cells. Cell Stem Cell. 2014;15(6):750-761. PMID: 25479750; PMCID: PMC4275050
– Zhu W, Zhao M, Mattapally S, Chen S, Zhang J. CCND2 Overexpression Enhances the Regenerative Potency of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Remuscularization of Injured Ventricle. Circulation Research. 2018 Jan 5;122(1):88-96. Epub 2017 Oct 10. PMID: 29018036; PMCID: PMC5756126
– Zhao M, Nakada Y, Wei Y, Bian W, Chu Y, Borovjagin AV, Xie M, Zhu W, Nguyen T, Zhou Y, Serpooshan V, Walcott GP, Zhang J. Cyclin D2 overexpression enhances the efficacy of human induced pluripotent stem cell-derived cardiomyocytes for myocardial recovery in a swine model of myocardial infarction. Circulation May 2021
– Kahn-Krell AM, Pretorius D, Ou J, Fast V, Litovsky S, Joel Berry J, Liu M, Zhang J. Bioreactor Suspension Culture: Differentiation and Production of Cardiomyocyte Spheroids From Human Induced Pluripotent Stem Cells. Frontiers in bioengineering-and-biotechnology
