Research Interests

Staphylococcus aureus chronic respiratory infections
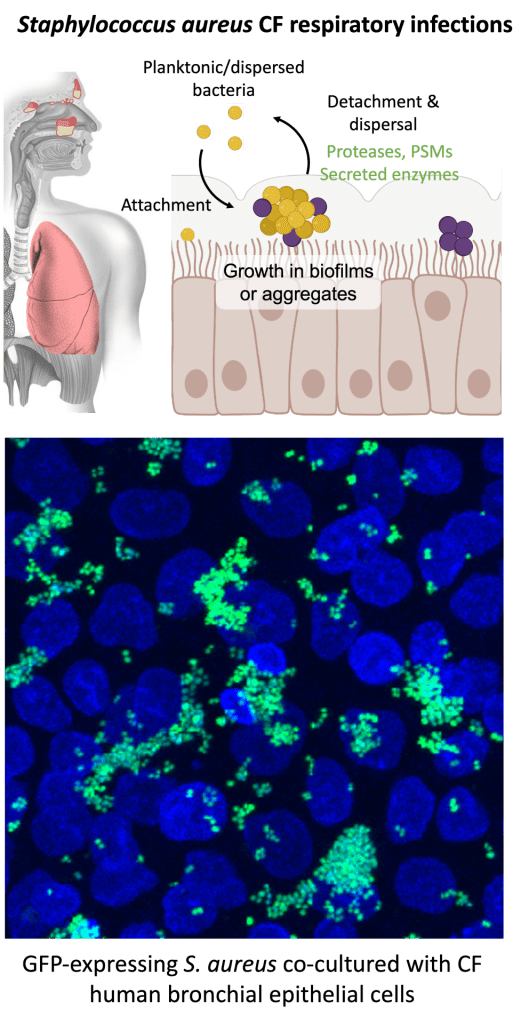
Staphylococcus aureus is the most frequently cultured bacterial pathogen in Cystic Fibrosis (CF) respiratory infections. S. aureus emerges as the dominant bacterial pathogen in pediatric populations, when methicillin-sensitive S. aureus (MSSA) is observed at a higher rate than methicillin-resistant S. aureus (MRSA). MRSA is identified more frequently in teenagers and adults with CF. The prevalence of S. aureus in CF is striking compared to the non-CF population, where rates of MRSA colonization are less than 1% compared to 20-25% of people with CF. Despite the high rate of S. aureus infection in CF, we know relatively little about S. aureus-host interactions in the CF respiratory tract and if there are CF-specific factors in the respiratory environment that influence S. aureus persistence and pathogenesis. The Kiedrowski Lab is interested in further defining S. aureus factors required for fitness and survival in the CF airways, an environment where dysregulation of the innate immune response, mucus secretion, antimicrobial peptide function, mucociliary clearance and oxygen availability have been well characterized.
People with CF experience periods of worse symptoms, called exacerbations, and frequently receive courses of antibiotic therapy. Frequent exposure to antibiotics can affect bacterial pathogens present in the airway and overall microbiome composition. The increase in and spread of antimicrobial resistance is a serious concern in treating CF respiratory infections, and drug resistance in the CF respiratory microbiome is associated with decreased lung function. How the respiratory microbiome is shaped in early CF disease and if interactions with other microbial community members influence S. aureus growth and virulence in CF infections is also an underexplored area. Projects in the lab are examining how antibiotic treatment affects bacterial populations and if interactions with other bacteria or the host affect S. aureus antimicrobial tolerance or the development of antibiotic resistance.
To study S. aureus and other bacteria that are commonly found in the respiratory tract, the lab uses an airway epithelial cell co-culture model. Culturing human bronchial or nasal epithelial cells in transwells with permeable filters allows us to remove liquid from the apical surface once the cell monolayer is confluent, signaling cells to polarize and establishing an air-liquid interface (ALI) that mimics the mucosal surface in the respiratory tract. S. aureus or other species of bacteria can then be inoculated onto the apical surface of ALI cultures and growth measured over time by counting viable colonies or monitoring fluorescent bacteria via microscopy. We can also collect airway surface liquid from the apical surface of ALI cultures to study how nutrients or secreted host factors supplied by the airway epithelium can influence bacterial growth, independent of direct contact with cells.
In collaboration with Dr. Susan Birket and her lab at UAB, we are also beginning to investigate S. aureus infection and pathogenesis in CF rat models.
Bacterial interspecies interactions in chronic sinus and upper respiratory diseases

Like CF respiratory infections, individuals with chronic rhinosinusitis (CRS), an inflammatory disease affecting the sinuses, also develop low-diversity bacterial communities dominated by genera associated with opportunistic pathogens, namely Staphylococcus, Haemohphilus, Pseudomonas, Streptococcus, Moraxella and Fusobacterium. Along with lung infections, people with CF also commonly experience chronic sinus and upper respiratory infections. The sinuses are hypothesized to play an important role in facilitating lower airway infections by providing a reservoir for pathogens to adapt to the host environment. Commensal bacteria in the airway microbiome are believed to play important roles in limiting respiratory pathogens by inhibiting pathogen colonization and invasion by inducing protective host responses, or in some instances by directly inhibiting pathogen growth by producing antimicrobials, competing for nutrients or blocking adhesion. Individuals who have a higher abundance of commensal bacteria and increased species diversity are commonly found to have less severe disease and better outcomes in CF and CRS. The genus Corynebacterium, which includes commensal species associated with a healthy microbiome, is almost completely absent in the upper airways of individuals afflicted with both CF and CRS but is highly abundant in healthy populations. Projects in the Kiedrowski Lab are investigating interactions of S. aureus and other CF and CRS pathogens with commensal species to gain a better understanding of factors that influence the development of dysbiosis in the sinuses and upper airways in chronic disease.
To determine the nature of chronic bacterial infections in the sinuses of CF CRS subjects, we can collect patient samples to evaluate bacterial biofilms ex vivo. Sinus biofilm material can be removed during sinus endoscopies, then fixed and embedded for cryosectioning. Using probes developed for specific 16S rRNA regions of S. aureus or other common pathogens like P. aeruginosa, we can then perform fluorescence in situ hybridization (FISH) staining to observe host cells and bacteria in sinus biofilm explants. Confocal microscopy revealed bacterial aggregates ranging from approximately 10-25um in size in sinus biofilm samples. A S. aureus probe identified S. aureus aggregates forming in samples from some subjects. General eubacterial labeling did not always overlap with species-specific probes, suggesting other unidentified species may also be present in mixed-species aggregates.
We are collaborating with Dr. Do-Yeon Cho in the UAB Department of Otolaryngology to continue to investigate bacterial chronic sinus infections in patient populations and using animal models of chronic sinus disease.
