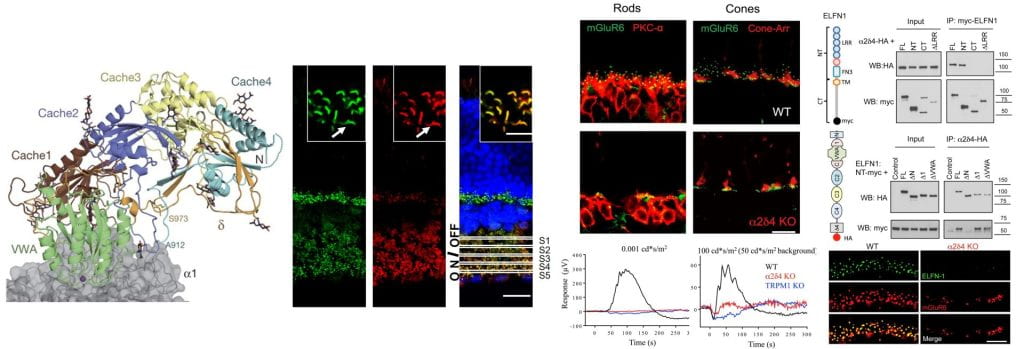
Faithful relay of information depends on the precisely adjusted spatial arrangement between presynaptic release sites and postsynaptic receptor signaling complex (Biederer et al., 2017; Haas et al., 2018; Tang et al., 2016). However, the molecular mechanism responsible for the pre- and post-synaptic alignment is not known. The α2δ proteins (α2δ1-4) are the only extracellular portion of the entire voltage-gated calcium channel (VGCC) complex, which is the central organizer of the presynaptic releasing machinery across the entire nervous system. Given that α2δs are multidomain proteins capable of mediating different protein-protein interactions and that all four members of α2δs have been recently shown as critical players for the formation and function of different synapses (Eroglu et al., Cell 2009; Fell et al., J Neurosci 2016; Pirone et al., J Neurosci 2014; Wang et al., Neuron 2017), we hypothesize that extracellular α2δ proteins serve as an universal mechanism underlying synaptic efficiency by ensuring the precise pre- and post-synaptic alignment.