Current Research
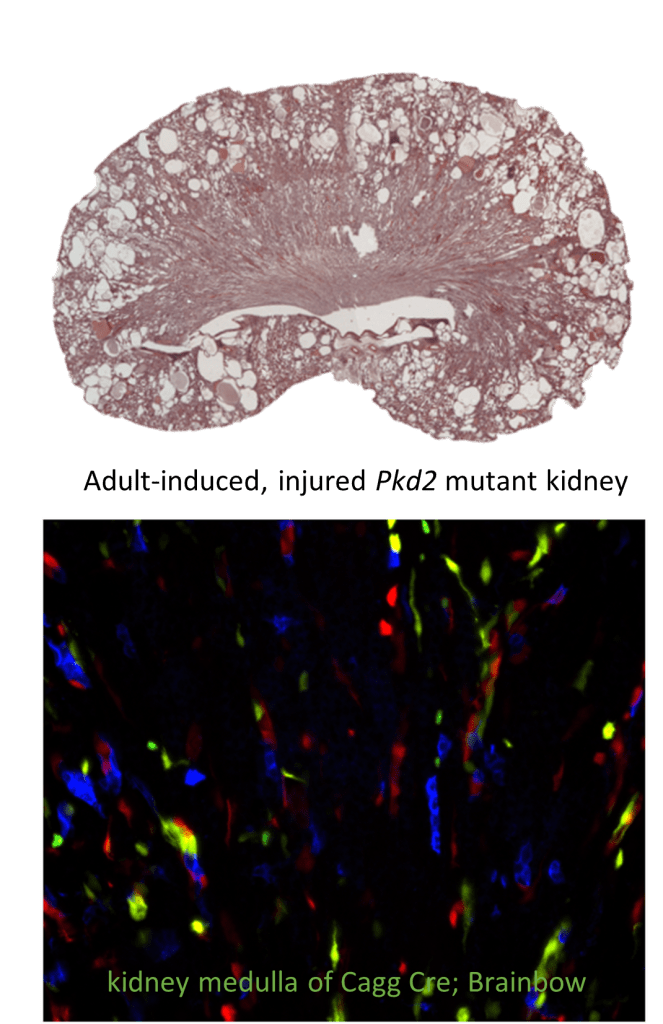
Injury Response Mediated Pathogenesis in Renal Ciliopathies
Primary cilia are present on most renal cells and their disruption leads to cyst formation in multiple model systems, as well as in humans. Despite their clinical importance, the function of the primary cilium in the kidney remains largely unknown. Recent data indicate that cilia disruption (Ift88 mutants) or mutations affecting ciliary function (Pkd1 or Pkd2 mutations) alter the ability of the kidney to undergo normal repair following kidney injury. Renal injury in these mutant backgrounds markedly accelerates the rate of cyst formation compared to non-injured cilia mutants. This project will define a novel requirement for cilia following injury, identify and test pathways involved in cilia-regulated injury response, evaluate whether injured cells directly contribute to the cysts, and determine how cilia dysfunction alters intercellular signaling pathways during cyst initiation and expansion.
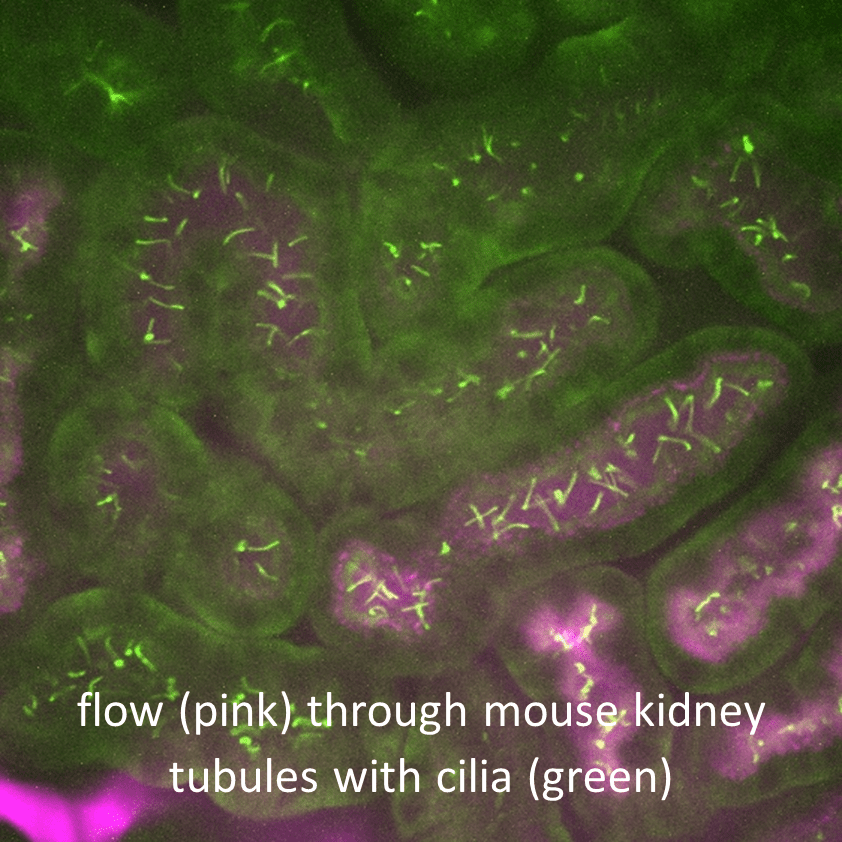
Intravital Imaging and Function of Cilia in Response to Kidney Injury
We are using mouse lines with fluorescently tagged cilia and bioreporters, intravital fluorescence confocal microscopy, and a surgically implanted abdominal window approach to image cilia in intact nephrons in living mice. Cilia are typically deflected in the direction of tubule flow under normal conditions. However, when flow is impaired, cilia behavior changes and they begin to oscillate and can elongate or regress. Impaired tubule flow also occurs following injury. Thus, one objective of this project is to investigate which in vivo conditions induce changes in cilia morphology, length, and number, ciliary Ca2+ fluctuations, and changes in transcriptional activity in response to normal flow, following injury, and during repair. Applying an intravital imaging strategy to address these questions is needed to understand renal cilia function and how the cilia respond to and regulate injury and repair mechanisms associated with cyst development.
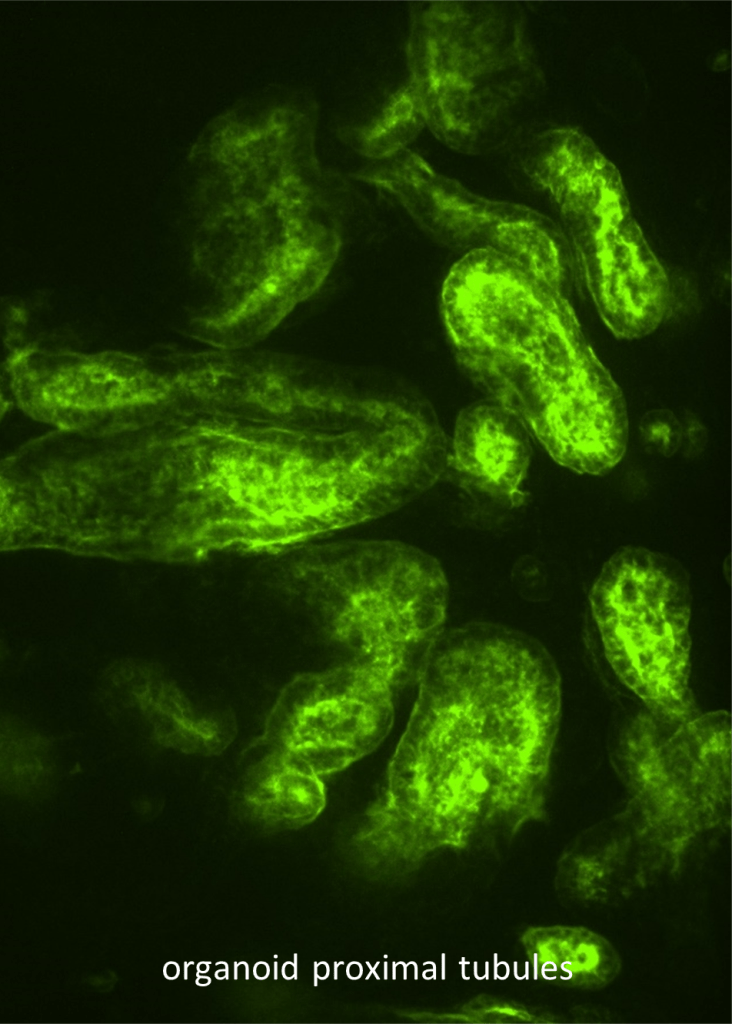
Nephrogenic Zone Organoids (NZOs) for Ex Vivo Organogenesis
Currently, thousands of patients with end-stage renal disease need kidney transplantation, but organ supply does not match demand. Ex vivo organogenesis has the potential to provide functional tissue for renal replacement therapy. We are currently using a technique that allows us to generate cellularly complex kidney organoids derived from mouse primary cells. This project aims to achieve integrated organoid tissue using selectively generated cell types. Specifically, we will identify conditions that will allow us to generate all nephron segments including glomeruli and proximal tubules with their associated interstitium and vasculature for engraftment. In this project, we will establish procedures to improve organoid patterning and identify factors that are intrinsic to the organoid or in the recipient kidney that promote tubule-tubule fusion and vasculature formation and maintenance.

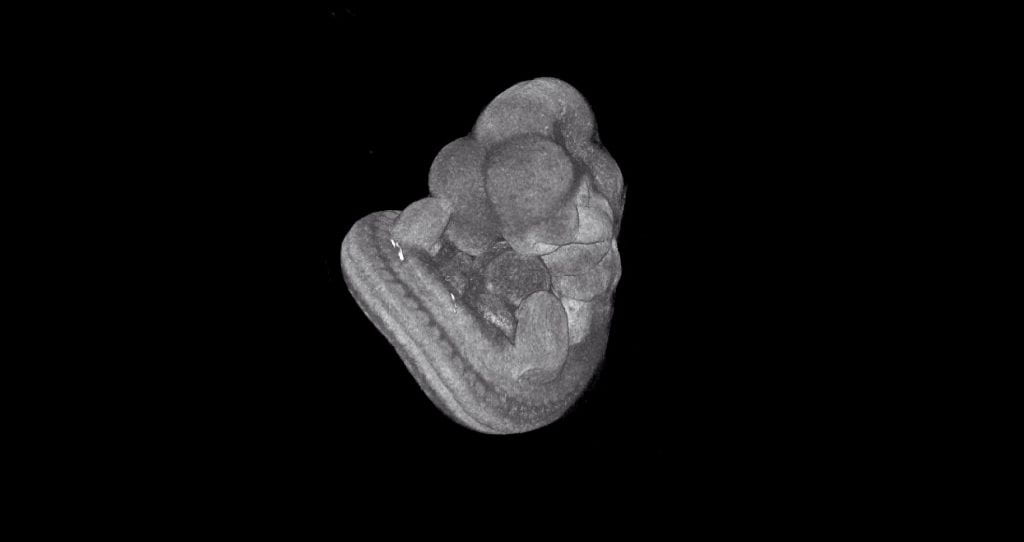
Ciliary Protein Functions in Development
We are using both null and conditional mouse mutant models to examine the function of various proteins required during mammalian development that are potentially related to ciliary biology. A variety of techniques including contrast-enhanced μCT, histology, and immunofluorescence staining are being used to analyze how loss of candidate cilia proteins affects embryogenesis due to possible functions of cilia. We have identified proteins essential for normal cardiac development, neural tube patterning, craniofacial development, and urogenital development and homeostasis and have begun to investigate how these proteins function in relation to primary cilia to regulate tissue morphogenesis.

