Yang Laboratory
Mission
Multiple myeloma (MM) is a largely incurable plasma cell malignancy. Myeloma preferentially grows in the bone marrow and frequently progresses from primary tumor sites to new, often distant, sites in bone (new bone sites), but the mechanisms driving MM dissemination and growth in new bone sites remain poorly understood. The goal of our lab is to investigate how MM cells alter bone marrow microenvironment in primary and new bone sites and how these changes feed back and influence tumor growth, dissemination, and chemo-resistance.
Research overview
Currently, our study focuses on:
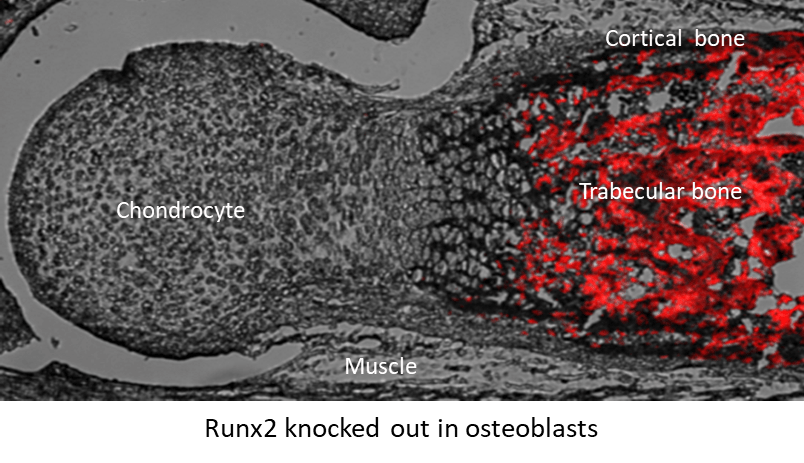
- To study the impact of the suppression of osteoblast-derived Runx2 (OB-Runx2) on the BM microenvironment and MM dissemination and drug resistance. We and others have demonstrated that many aggressive bone cancers, including MM, inhibit osteoblastogenesis and bone formation via the suppression of Runx2 expression in osteoblasts. Importantly, we have discovered that MM cells in primary sites secrete soluble factors to suppress OB-Runx2 expression at new bone sites, prior to the arrival of metastatic tumor cells. However, it is unknown whether and how OB-Runx2 suppression in new bone sites regulates MM metastasis to and progression in these areas. Our lab has developed a unique syngenic animal model of murine MM, in which Runx2 is specifically deleted in OBs (Runx2OB-/-). We are using a variety of molecular and biochemical approaches and a novel in vivo approach (Runx2OB-/- model) to test the influences of OB-Runx2 deficiency in bone microenvironment and in MM growth, metastasis and drug resistance.
- To investigate how osteocyte apoptosis induced by MM cells feeds back and influences MM progression and chemoresistance. Our previous studies have demonstrated that MM cells induce death and apoptosis of osteocytes in distant bones prior to the arrival of metastatic cells. To investigate the effects of osteocyte apoptosis on MM dissemination and chemo-resistance, we have developed two mouse models of MM, a diphtheria toxin receptor model (DTR) and bone unloading model (hind limb suspension) that allow osteocyte apoptosis to be induced by either diphtheria toxin (DT) injection or hind limb suspension, respectively. Using these two animal models, we are studying the impact of osteocyte apoptosis on bone microenvironment and consequently on MM metastasis to distant new bone sites.
