Alpha-synuclein (αsyn) is the key protein that is aggregated in PD patient brains, but how α-syn causes neurodegeneration is not well understood. αSyn was first linked to PD through genetic studies of rare forms of autosomal dominant PD, and was later recognized to play a key role in sporadic PD when it was discovered as the key component in Lewy Bodies, the pathological hallmark of PD. Increased αsyn levels and aggregation are thought to be important to its toxicity. Recent evidence points to the ability of αsyn to spread from cell to cell in the nervous system, causing misfolding of endogenous αsyn in synaptically-connected cells.
Key questions:
- What are the mechanisms that regulate the release and uptake of αsyn?
- What cellular pathways are involved in the release of toxic forms of αsyn
- How can the spread and propagation of αsyn be halted in disease?
We use two main models to examine these questions regarding the cell-to-cell transmission of αsyn:
- The αsyn paracrine model
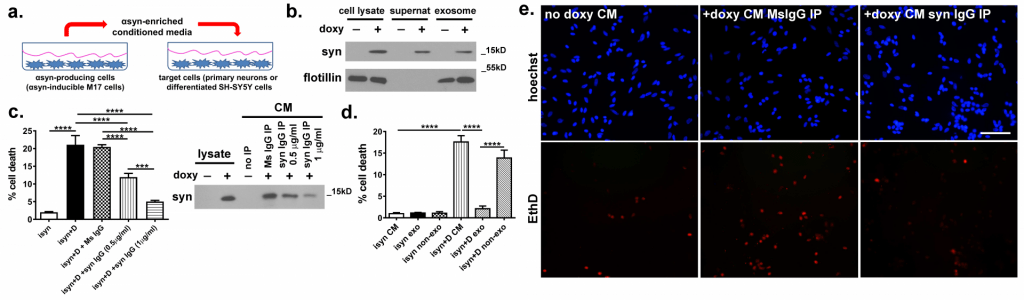
This model uses a doxycycline-inducible neuroblastoma cell line that produces high levels of human αsyn upon induction. This αsyn is actively released into the conditioned media and is toxic when transferred to separately cultured primary neurons. This model allows us to measure protein secretion, uptake, and protein degradation along with examination of biophysical properties of released αsyn.
- The preformed fibril (PFF) model
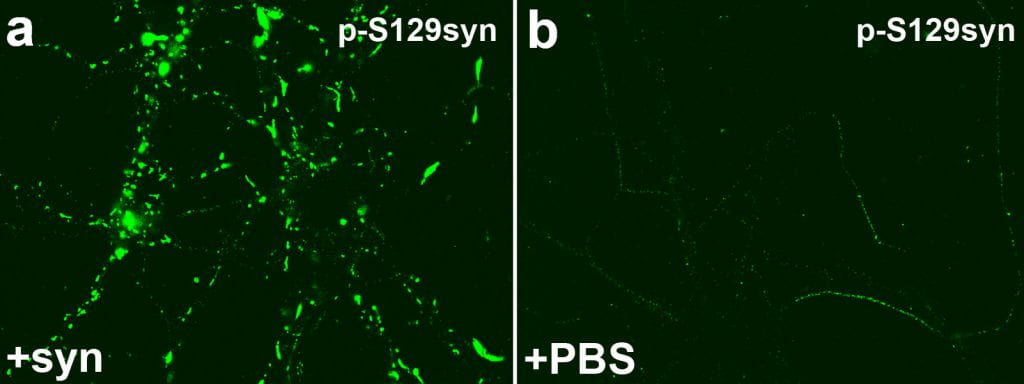
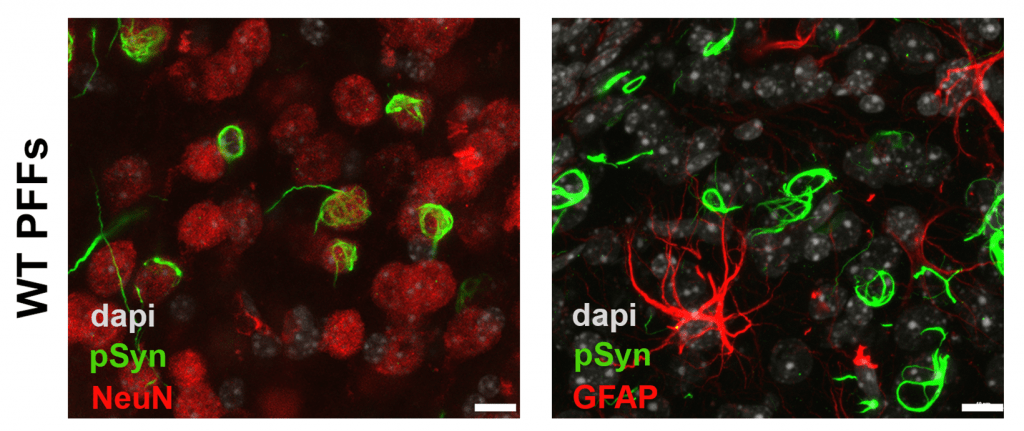
This model uses fibrillized forms of recombinant αsyn which induce misfolding and aggregation of endogenous αsyn in neurons in culture or in vivo.