RNA Regulation of Neuroinflammation
My laboratory has a long-term interest in the RNA regulation of cytokine and chemokine expression in glial that controls neuroinflammatory responses in acute and acute CNS disorders including amyotrophic lateral sclerosis (ALS), spinal cord injury, neuropathic pain and stroke. We have focused on HuR, a major positive regulator of cytokine mRNAs, and its role in microglia and astrocytes to promote neuroinflammation. When activated, HuR translocates to the cytoplasm and promotes the expression of pro-inflammatory cytokines by augmenting the stability and translational efficiency of the cytokine mRNA. Our team has recently developed a class of small molecule HuR inhibitors that blocks cytoplasmic translocation and may have promise in treating disorders driven by neuroinflammation. Our models also extend to malignant glioma as HuR plays an important role in tumor progression through its regulation of the secretome in glioma-associated macrophages and microglia.

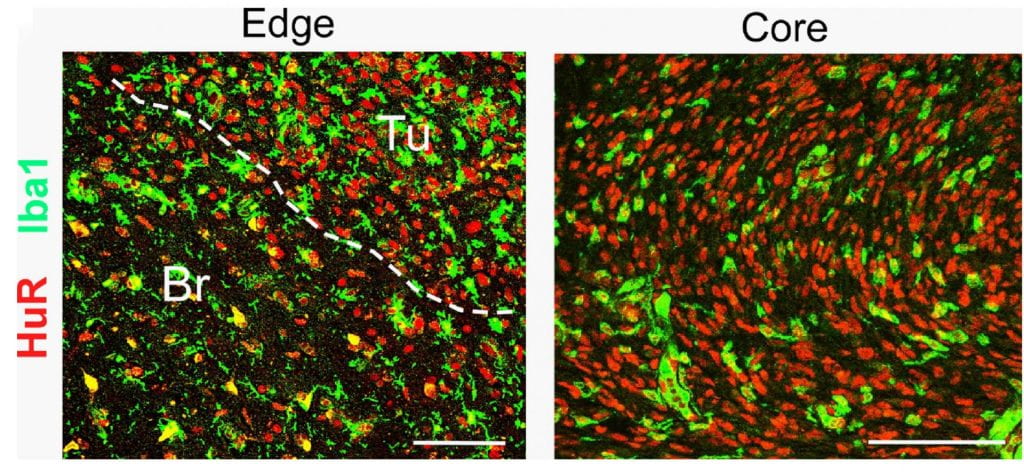
Human ALS spinal cord showing cytoplasmic translocation of HuR in activated Iba1+ microglia (merged yellow color)–Glia, 2017.
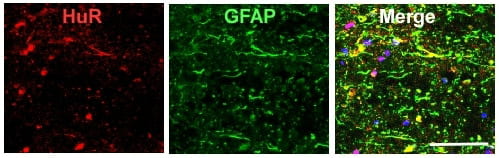
Mouse spinal cord in the epicenter of a mid-thoracic contusion injury at 24 h. HuR is translocated to the cytoplasm in GFAP+ astrocytes (merged yellow color)–J Neurotrauma, 2017.
Peripheral neuromuscular pathology in ALS
The second area on interest centers on the peripheral neuromuscular system in ALS and molecular dysregulation that may accelerate disease progression. We and collaborators have identified key inflammatory pathways driven by mast cells, macrophages, and terminal Schwann cells that are implicated in disease progression. Using ALS patient-derived tissue samples and the SOD1 mouse, we have identified a molecular network of proteins and non-coding RNAs in muscle that is activated in the early preclinical stages of disease and increases with disease progression. Investigation of this network is underway but closely ties in with inflammatory responses that drive disease progression. Further characterization of this network has the promise of identifying new therapeutic targets in ALS and biomarkers that can help track disease progression. Masitinib, for example, is a tyrosine kinase inhibitor that blocks these inflammatory responses, is now in a Phase III study for patients with ALS.
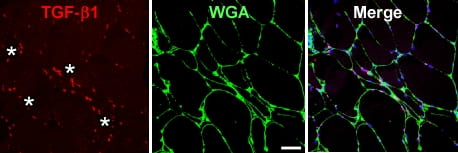
TGF-b1 + cells surround a group of atrophic muscle fibers (asterisks) in a muscle biopsy from a patient with ALS (PloS One, 2015)
Glioblastoma
This tumor provided the original in vitro model in our laboratory to study posttranscriptional gene regulation by members of the Hu family (HuR, Helen-1, HuC and HuD). The major focus has been on the role of HuR in promoting tumor progression through upregulation of growth-associated and anti-apoptotic factors in glioma cells and glioma stem cells including VEGF, BcL2, CIAP, IL-6 and IL-8. More recently we have found that HuR promotes the expression of factors secreted by glioma-associated microglia and macrophages that promote tumor progression.