Some key questions in the lab are:
- What drives the unique biological and clinical behavior of tumors with TERT promoter mutations?
- What are the unique clinical vulnerabilities of tumors with TERT promoter mutations?
- Can existing therapeutics be redeployed in new clinical settings for tumors with TERT promoter mutations, i.e. do these mutations offer a precision medicine opportunity?
- What drives the poorer patient outcomes that often associate with these mutations?
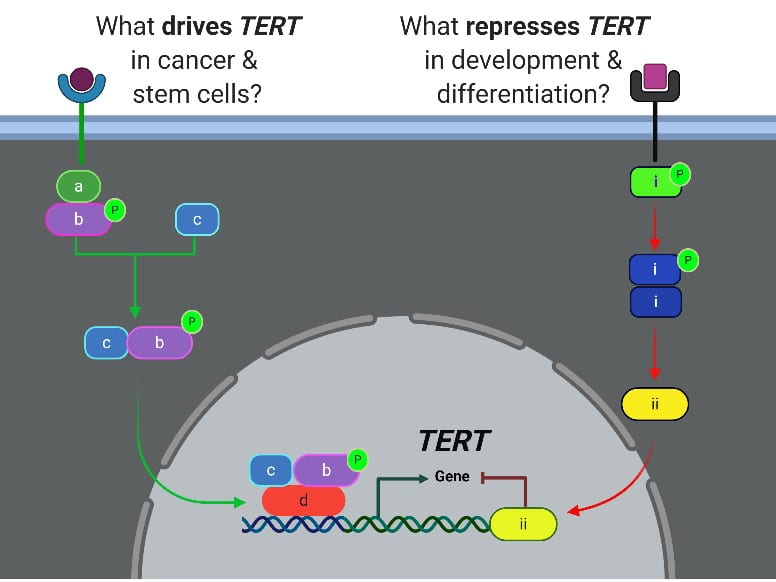
- What are the molecular mechanisms driving telomerase in cancer cells and stem cells?
- What represses telomerase upon differentiation of stem cells to mature cell types?
- What factors are required to de-repress telomerase during induction of pluripotency and oncogenesis?
- Precisely how does telomerase-mediated telomere maintenance impact cellular function?
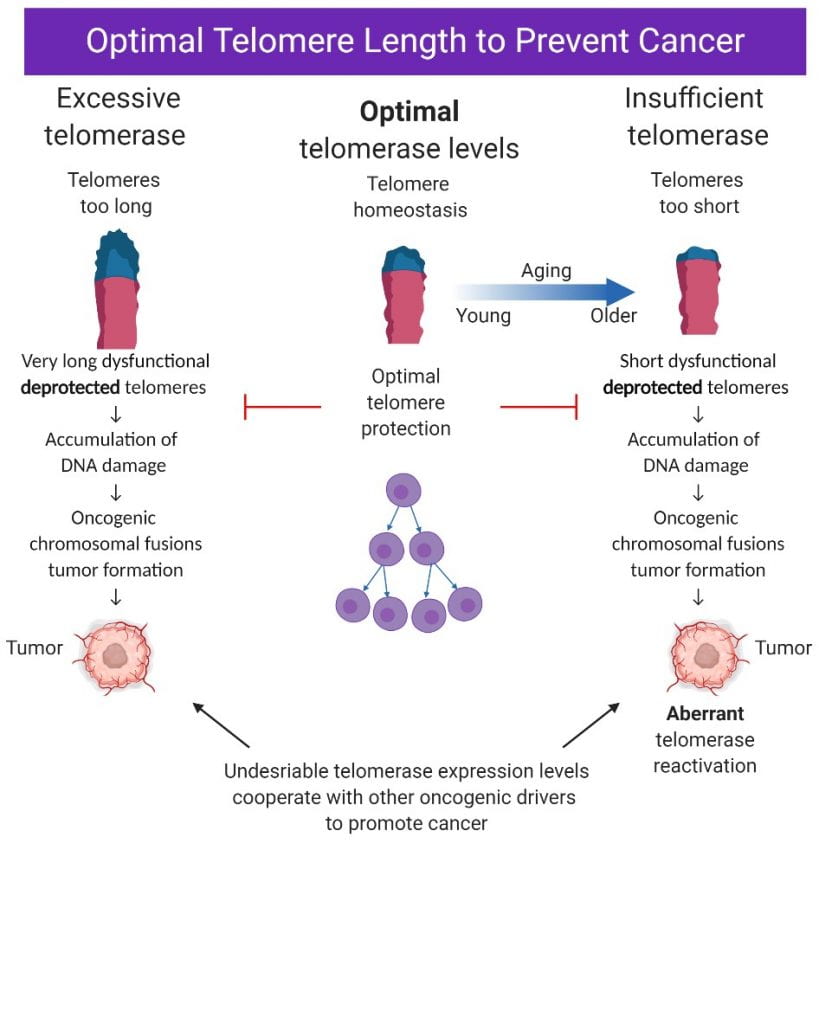
Unchecked, tumor cells divide unceasingly. This perpetual division contributes significantly to cancer’s lethal nature. Around 90% of all cancers rely on a common set of genes to support this indefinite growth; these genes code for the enzyme telomerase. Studies indicate that inhibiting telomerase in cancer cells can, in many instances, restrict tumor development and growth. The normal function of telomerase is to maintain telomere length in stem cells, but the enzyme is aberrantly expressed in most cancer cells1–4 providing them with the critical ability to maintain their telomeres.
In contrast to cancer, hypomorphic (partial loss of function) mutations in telomerase can cause severe, life-threatening diseases such as dyskeratosis congenita, aplastic anemia and idiopathic pulmonary fibrosis. These diseases are caused by the failure of telomerase to adequately maintain telomere length, leading to catastrophic organ dysfunction as individuals age. Understanding how to improve or rescue telomerase function for these patients may provide therapeutic relief5–7.
Telomerase activation in cancer cooperates with other oncogenic ‘driver genes’ allowing for the indefinite proliferation of tumor cells. TERT encodes the catalytic subunit of the telomerase enzyme. About 20% of cancers harbor activating mutations in the proximal promoter for TERT8–10. These TERT promoter (TERTp) mutations are heterozygous and consequently drive allele-specific expression11,12. They are especially prevalent in glioblastomas, melanomas, myxoid liposarcomas, liver cancers, thyroid cancers, and bladder cancers10,13,14 but are also present at lower frequencies in many other cancer types. For unknown reasons TERTp mutations frequently associate with poorer patient survival15–24. Importantly, the specific transcription factor GABPb1L was shown to regulate survival in a TERT-dependent manner25 in mice with TERTp mutant xenografts of glioblastoma, demonstrating that these factors drive aggressiveness in an established model of brain cancer.
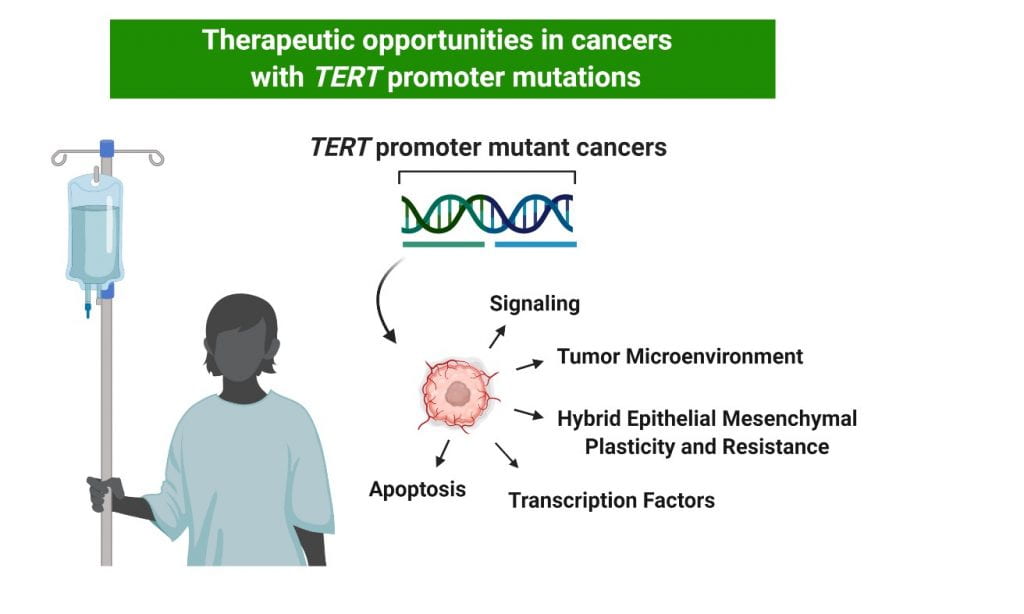
Building on excellent work from others, we have identified that TERTp mutations strongly associate with a hybrid epithelial/mesenchymal (E/M) state and MAPK/RAS driven gene and protein expression signatures26. These signatures predict that TERTp mutants may exhibit phenotypic plasticity and enhanced therapeutic resistance27,28. We have found that these cancers display selective sensitivity to therapeutic agents already in clinical use for other indications26. These findings encourage the use of the TERTp mutation as a biomarker for the novel deployment of existing therapeutic approaches and for the development of new treatment regimens that target the cooperativity between the hybrid E-M state and RAS-driven programs.
Greater than 99% of our body’s cells do not express telomerase. However, telomerase is expressed in stem cells and helps these cells to proliferate throughout our adult life. Loss of telomerase expression in stem cells as we get older contributes to our overall aging. As stem cells become less proliferative, the tissues that they supply with new cells begin to lose their normal function29,30. This age-associated tissue dysfunction can, to some degree, be rescued by re-expression of telomerase31–33. The factors that promote telomerase expression in stem cells, that drive telomerase repression in differentiated cells or in aging, or telomerase reactivation during induction of pluripotency, are not well understood.