Contents
Enhanced Skeletal Muscle Proteostasis as a Determinant of Central Nervous System Aging and Disease.
Our work straddles the fields of neuroscience and metabolism, using an integrated physiology approach to understand diseases of the Central Nervous System (CNS). We have recently discovered novel skeletal muscle signaling pathways that can modulate the rate at which the brain ages, and we are currently working on identifying these muscle-originating signals that enhance CNS function and characterize their impact on healthy CNS aging and Alzheimer’s disease (AD) pathogenesis.
Our goal is to identify novel therapeutic diagnostics and targets for the treatment of neurodegenerative diseases, radically transforming our therapeutic approach by a) uncovering novel muscle-specific druggable targets, and b) facilitating therapeutic compound delivery for pre-clinical AD mouse trials guided by these results.
Healthy Brain Aging
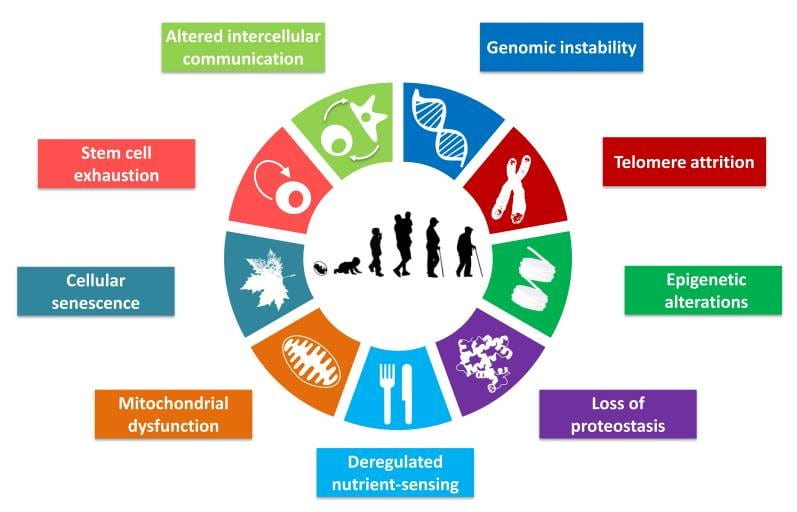
Lopez-Otin et al, Cell. 2013 Jun 6; 153(6): 1194–1217.

Aging is a multifactorial, complex process driven by a variety of cellular processes. In the wheel on the right, we highligh the nine most-common hallmarks of all aging tissues. The brain is no different, undergoing similar alterations throughout our lifespan. Our lab is particularly interested in understanding how loss of protein quality control (proteostasis, in purple) and altered intercellular communication (green) intersect. We believe aging is a integrated process, and that modulation of aging phenotypes in one tissue (skeletal muscle) may have important effects in the CNS.
Proteostasis (protein homeostasis) is essential for cell health and viability, and involves complex and highly conserved networks that regulate protein translation, protein folding, and protein degradation. A decline in proteostasis function is one of the features of aging tissues, particularly of the central nervous system (CNS). Indeed, the aging brain is particularly sensitive to proteotoxic stress, as demonstrated by the high number of age-associated neurodegenerative disorders characterized by protein misfolding and aggregation, including Alzheimer’s disease (AD). The regulation of non-cell autonomous proteostasis has recently arisen as a novel mechanism for the modulation of systemic homeostasis in worms and flies, and is postulated to have important organismal effects on metabolism and aging. However, to date, there are no studies addressing the existence and activity of these pathways in mammals, and their potential effects on the aging brain. We have recently discovered that enhanced skeletal muscle proteostasis function can significantly ameliorate proteotoxicity in the CNS and also improve cognition and memory in aging mice. Our current work aims to characterize these targets and validate their therapeutic potential for diseases of the aging CNS.
Alzheimer’s Disease

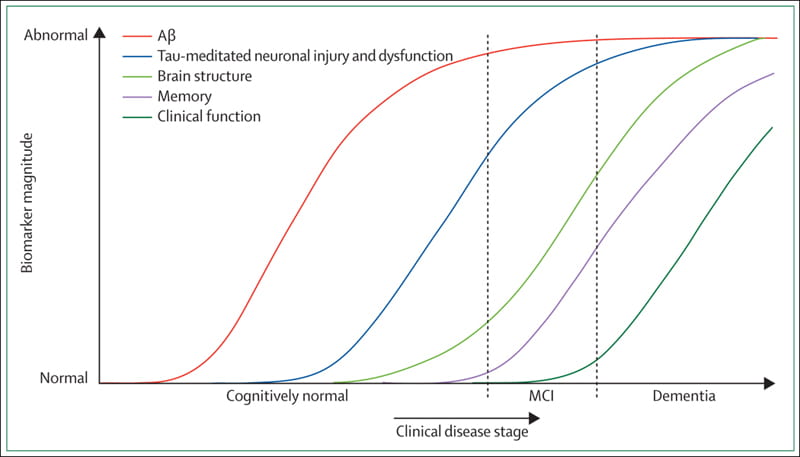
Our interest in novel mechanisms of CNS aging provided a natural pivot into the field of Alzheimer’s disease (AD). As shown on the graph on the right, several of the systemic biomarkers currently used for AD-diagnosis, including AB-42 elevations and accumulation of hyperphosphorylated tau, appear many years before clinical onset of neurological symptoms.
Although systemic metabolic deficiencies and weight loss are clinical features of AD, and are often described years before cognitive impairment, the contribution of skeletal muscle dysfunction to AD pathogenesis (if any) remains largely unexplored.
We are thus currently examining the potential neuroprotective effects of enhancing muscle proteostasis in AD, and characterizing transcriptomic changes in AD-skeletal muscle. This represents a novel approach to AD as a systemic disease and has the potential to uncover novel biomarkers and therapeutic targets.
Niche vs. Self: Intrinsic Mechanisms of Skeletal Muscle Aging
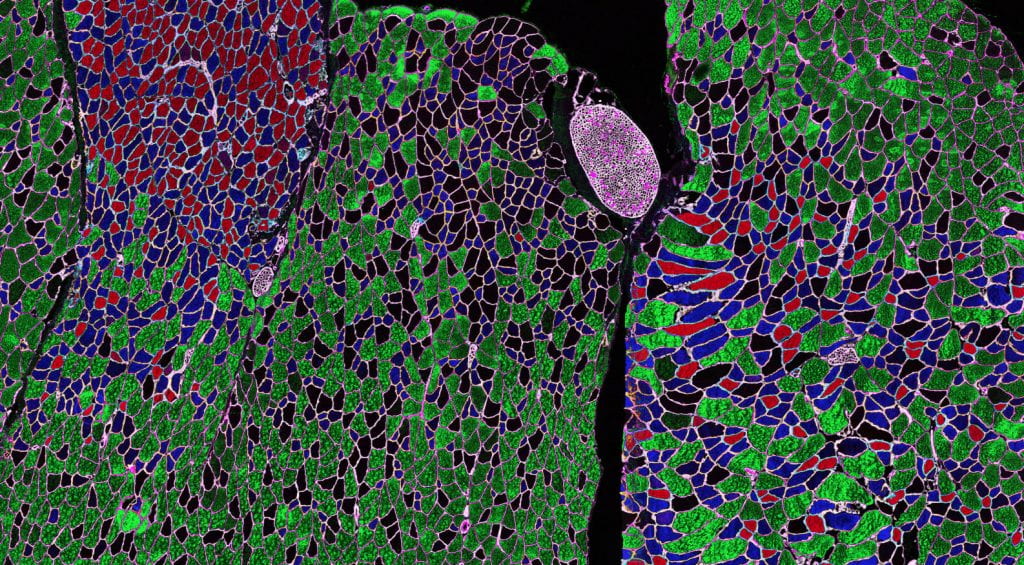
Despite recent advances in understanding the molecular mechanisms of aging, the cross-talk between age- related decline of global tissue function and stem cell exhaustion remain poorly understood. Skeletal muscle, accounting for ~40% of an adult (lean) human’s body weight, has tremendous implications for quality of life during aging.
The decline in size and function of the skeletal muscle stem cell (MuSC) pool during aging has been well characterized and is suggested to be a driving force in the age-related decline in muscle health. In healthy young organisms, MuSCs remain quiescent but are poised to rapidly activate, proliferate, and differentiate in response to muscle injury. A subset of proliferating MuSCs self-renew and retain stem cell properties to return to quiescence at the completion of the regenerative process.
MuSC dysfunction in aged muscle can be attributed to circulatory factors and changes in the local microenvironment (niche) as well as several intrinsically deregulated signaling, metabolic, and epigenetic pathways. Importantly, the relative contribution of cell autonomous (intrinsic) vs. non-cell autonomous (extrinsic) factors of MuSC decline during aging is a topic of much debate. Our lab has identified potent niche effects in our models of activated muscle-autophagy, including improved regeneration responses and MuSC numbers in aging muscle. We are currently investigating the mechanisms underlying these effects.