Ralph Sanderson Lab

Research Overview
The overall goal of our lab is to determine how heparan sulfate proteoglycans and heparanase act to regulate the tumor microenvironment and how this promotes tumor growth, angiogenesis, metastasis and osteolysis. We are recognized experts in this area of research and have had uninterrupted NIH funding for our studies for the past 30 years. Currently, our primary focus is on heparanase, an enzyme that degrades heparan sulfate chains and promotes the aggressive behavior of cancer. We have discovered that by regulating the expression of a number of key genes and by enhancing shedding of the cell surface proteoglycan syndecan-1, heparanase acts as a strong promoter of the aggressive tumor phenotype. Recently we have also shown that heparanase promotes stemness of multiple myeloma tumor cells. Work from our lab was instrumental in establishing that heparanase acts as a master regulator of myeloma progression, metastasis and chemoresistance and a viable target for therapy. We successfully completed preclinical studies in animal models of myeloma with the novel heparanase inhibitor Roneparstat (SST0001). This work formed the basis for successful completion of phase I clinical trial in myeloma patients.
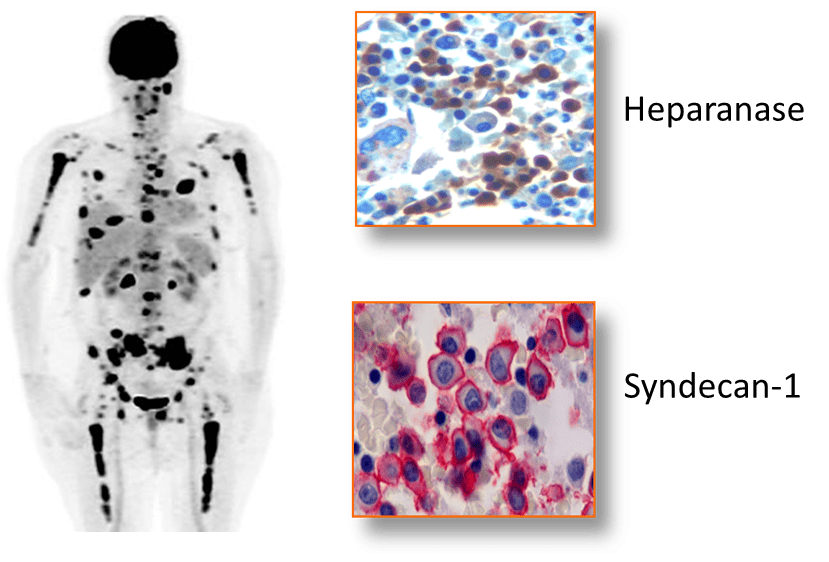
Myeloma is a devastating cancer that resides predominantly within the bone marrow where it proliferates, metastasizes and degrades bone. This figure shows a FDG-PET scan of a myeloma patient having multiple tumor lesions within long bones, vertebra and ribs (Eu. J. Radiol. 83:2203). Bone marrow biopsies from myeloma tumor lesions demonstrate positive immunostaining for heparanase and syndecan-1 (Cancer Res. 63:8749, Mod. Pathol. 14:1052).
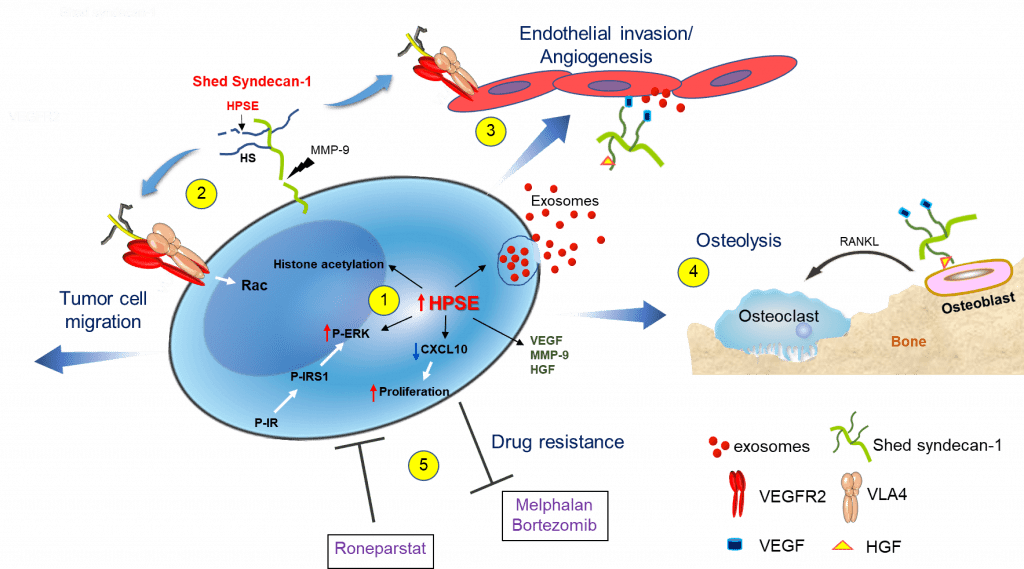
This figure depicts our discoveries establishing that heparanase triggers multiple pathways that drive myeloma progression. (1) Enhanced expression of heparanase by myeloma cells: augments gene transcription by enhancing acetylation of histones, stimulates exosome biogenesis by trimming the heparan sulfate chains of syndecan-1 thereby priming formation of the syndecan-syntenin-ALIX complex, downregulates CXCL10 causing increased tumor cell proliferation and activates ERK via the insulin signaling pathway resulting in enhanced expression of MMP-9 and VEGF. (2) Shedding of syndecan-1 from the myeloma surface is driven by the heparanase-mediated trimming of heparan sulfate and by the increase in MMP-9 secretion. The shed syndecan-1 complexes with VLA-4 and VEGFR2 on the tumor cell surface stimulating Rac signaling and resulting in cell migration/invasion. (3) Via the same mechanism as in tumor cells, shed syndecan-1 initiates Rac signaling in endothelial cells that promotes angiogenesis. Increased angiogenesis also occurs when angiogenic growth factors (VEGF, HGF) bound to shed syndecan-1 heparan sulfate chains activate receptors on endothelial cells and when exosomes bearing VEGF, HGF and heparanase cargo dock with endothelial cells. (4) Similarly, HGF bound to shed syndecan-1 activates the cMet receptor on osteoblasts that via an IL-11 feedback mechanism increases RANKL secretion leading to osteoclast activation and osteolysis. (5) Myeloma cells having elevated heparanase expression exhibit resistance to commonly used anti-myeloma drugs, including proteasome inhibitors (bortezomib, carfilzomib) and the alkylating agent melphalan. Conversely, exposure of cells to the heparanase inhibitor Roneparstat blocks the multiple pathways that are stimulated by heparanase (e.g., syndecan shedding, angiogenesis) resulting in decreased drug resistance and inhibition of myeloma growth in vivo. Figure and legend from Adv. Exp. Med. Biol. 1221:331.
Mission
- To explore the role of heparanase in multiple myeloma progression, metastasis and chemoresistance and to translate these discoveries into anti-cancer therapies.
- To provide an intellectually rich, challenging, and safe environment for employees and trainees of all backgrounds to acquire the skills necessary to become productive members of the scientific community.
- To promote the importance of the study of proteoglycans, heparanase and extracellular vesicles in cancer and other diseases.
