Contents
Questions that guide us:
- What enables a cancer cell to metastasize?
- Can we interfere in that process?
What have we learned so far:
- Signaling pathways that play a role in normal tissue and organ development are
reactivated and distorted by cancer cells. - Cancer cells are de-differentiated or stem-like in their behavior.
- The cells that can face various stress factors from the tumor microenvironment are most effective in successful metastatic colonization.
What do we focus on:
- Gland: Mammary glands and their development
- Developmental signaling pathway: Wnt/ß-catenin pathway
- Stress Factors: Hypoxia and heat shock
- Cellular Process: EMT and Invasion
- Intracellular compartment: The nucleolus
Ongoing Research:
A. Discovering how breast cancer cells respond to extraneous stimuli by modulating
ribosome biogenesis
- Chronic hypoxia is frequently observed in solid tumors. We recently showed that chronic hypoxia contributes to genesis of ribosomes with specialized epitranscriptomic (2’-O Me) marks following increased RNA Pol I activity. This observation supports
existence of specialized ribosomes.
- Triple negative breast cancer (TNBC) cells have increased Wnt/ß-catenin signaling. TNBC cells also have increased number of nucleoli per cell. We showed that TNBCs have elevated ribosome biogenesis. The majority of this elevated ribosome
biogenesis is supported by activated Wnt/ß-catenin signaling.
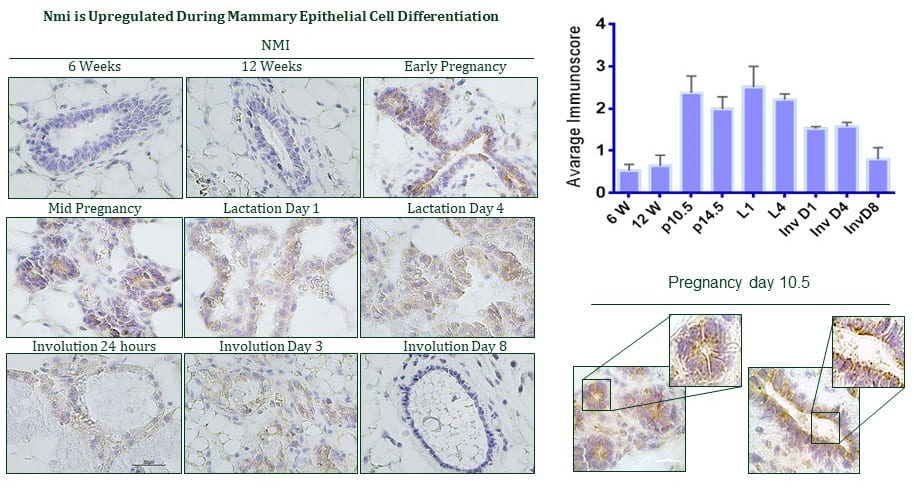
B. Understanding how the loss N-Myc Interactor (NMI), a normal mammary luminal cell differentiation regulator protein, aids tumor progression and metastasis.
- NMI is a protein involved in normal mammary development, however its expression is severely compromised in normal mammary development. We made mammary specific knockout of NMI gene. Using this genetically engineered mouse (GEM) model we found out that NMI restricts Wnt/ß-catenin signaling in differentiated mammary luminal cells. Loss of NMI in cancer cells render them highly invasive and metastatic due to
unrestricted Wnt/ß-catenin signaling and increased EMT.