 Read UAB News article titled “Stealth pig cells may hold key to treating diabetes in humans,” featuring CNMB member, Dr. Eugenia Kharlempieva.
Read UAB News article titled “Stealth pig cells may hold key to treating diabetes in humans,” featuring CNMB member, Dr. Eugenia Kharlempieva.
CNMB NSF NIH News
NIH Funds UAB-Vivo Biosciences Research Team in Vascular Grafts
NIH Funds UAB-Vivo Biosciences Research Team in Vascular Grafts

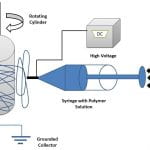
NIH-National Center for Advancing Translational Sciences (NCATS) has funded a UAB-Vivo biosciences research team for conducting translational research on vascular grafts led by Principal Investigator Yogesh K. Vohra, PhD. This Phase-I award of $192,314 is under the Small Business Technology Transfer (STTR) program. Approximately 1.4 million surgical procedures that require arterial prostheses are performed annually in the US due to peripheral vascular disease and ischemic heart disease; approximately 500,000 of these are coronary artery bypass operations. The current commercially available synthetic grafts have not been found suitable for small vessel (<6 mm) applications, with a <50% patency rate at 12 months. Disadvantages of prosthetic grafts include increased risk of thrombosis, poor tissue response and integration and poor biomechanics of graft, leading to additional interventions. Because there are no acceptable synthetic prostheses for small-diameter blood vessels, there is a huge demand for tissue engineered vascular grafts, especially small diameter vascular grafts for coronary replacement. The overall goal of this new program is the development and evaluation of a novel functionally-graded biohybrid vascular graft for small diameter for coronary bypass applications. Tissue engineered vascular constructs developed to date have been engineered mostly using synthetic and animal-derived biomaterials and require pre-seeding of cells before implantation to overcome the complications of prosthetic vascular grafts. However, these biohybrid systems exhibit many limitations including poor cellular adhesion, inadequate biomechanical and functional properties. The UAB research group has recently demonstrated an in vitro regenerated human endothelium on functionally-layered polymeric scaffolds containing bioactive proteins (Figure below). Moreover, Vivo Biosciences has developed a unique human biomatrix (HuBiogel™) that allows viable tissue constructs by cultivating single or multiple cell types. The research team will combine our functionally-layered graft strategy with this physiological HuBiogel culture technology for fabricating an advanced 3D vascular construct demonstrating enhanced lumen endothelialization and biocompatibility. Our research team includes Dr. Yogesh Vohra (PI, UAB Physics/College of Arts and Sciences) Dr. Vinoy Thomas (UAB MSE/School of Engineering) Dr. Steven Pogwizd (School of Medicine – Cardiovascular Disease)
and Dr. Raj Singh (President -Vivo Biosciences).
Dr. Eugenia Kharlampieva Awarded a NSF Faculty Early Career Development Award
NSF Faculty Early Career Development Award (CAREER)
 “CNMB member and a faculty member in the UAB Department of Chemistry Dr. Eugenia Kharlampieva has been awarded a National Science Foundation (NSF) Faculty Early Career Development Award entitled “CAREER: Shape Responses of Ultrathin Hydrogel Microcapsules” for a five year period 2014-2019. This is one of the National Science Foundation’s most prestigious awards in support of junior faculty who exemplify the role of teacher-scholars through outstanding research, excellent education and the integration of education and research within the context of the mission of their organizations”
“CNMB member and a faculty member in the UAB Department of Chemistry Dr. Eugenia Kharlampieva has been awarded a National Science Foundation (NSF) Faculty Early Career Development Award entitled “CAREER: Shape Responses of Ultrathin Hydrogel Microcapsules” for a five year period 2014-2019. This is one of the National Science Foundation’s most prestigious awards in support of junior faculty who exemplify the role of teacher-scholars through outstanding research, excellent education and the integration of education and research within the context of the mission of their organizations”
Dr. Claudiu T Lungu Awarded a National Institute for Occupational Safety & Health Grant
CNMB Member Dr. Claudiu T Lungu Awarded a National Institute for Occupational Safety & Health Grant (CDC/DHHS R21, Total amount for two years: 09/01/2013 – 08/31/2015: $398,672)
 The goal of this study is to develop a new technique that allows accurate sampling of gases and vapors at low levels.
The goal of this study is to develop a new technique that allows accurate sampling of gases and vapors at low levels.
Dr. Lungu is investigating a new process of using pulsed visible light to release chemicals from new substrates based on carbon nanotubes. These substrates will be used in air sampling devices for volatile organic compounds and gases for workplace or environmental monitoring. This research could potential lead to a new generation of air samplers that may be faster, cheaper, and more sensitive than the currently available models.
Dr. Eugenia Kharlampieva Awarded a NSF Biomaterials Grant
Dr. Eugenia Kharlampieva Awarded a National Science Foundation Biomaterials Grant
Immunomodulatory Ultrathin Multilayer Coatings for Pancreatic islet Transplantation NSF-DMR1306110, program of Biomaterials (BMAT)
 This grant will supports the development of a novel type of cytoprotective material with controlled immunomodulatory and inflammatory responses to be used for cell-basedtransplantation therapy for diabetic recipients. This project is in collaboration between departments of Chemistry (Eugenia Kharlampieva, PI) and Microbiology (Hubert Tse, coPI). Although transplantation of pancreatic islet cells has emerged as a promising treatment for Type 1 diabetes, its clinical application remains limited due to adverse effects of immunosuppression and declining allograft function. The awarded project will develop a preclinical approach to preserve islet viability and function during culturing and transplantation by protecting pancreatic islets (cell clusters) with a novel polymer coating. These coatings will be designed through hydrogen-bonded assembly of cytocompatible macromolecules with antioxidant and anti-inflammatory characteristics. This project is particularly timely since current islet encapsulation systems are challenging for transplantations due to high cytotoxicity and the requirement for large injection volumes. The design of novel immunoprotective materials will open new prospects for developing biomaterials with unique characteristics having applications in various bio-related areas such as bioengineering and tissue engineering. The awarded project will develop interdisciplinary collaborative research which should stimulate awareness of the needs of the UAB biomedical research community for specialized polymer-based biomaterials as novel platforms for cell transplantation therapy.
This grant will supports the development of a novel type of cytoprotective material with controlled immunomodulatory and inflammatory responses to be used for cell-basedtransplantation therapy for diabetic recipients. This project is in collaboration between departments of Chemistry (Eugenia Kharlampieva, PI) and Microbiology (Hubert Tse, coPI). Although transplantation of pancreatic islet cells has emerged as a promising treatment for Type 1 diabetes, its clinical application remains limited due to adverse effects of immunosuppression and declining allograft function. The awarded project will develop a preclinical approach to preserve islet viability and function during culturing and transplantation by protecting pancreatic islets (cell clusters) with a novel polymer coating. These coatings will be designed through hydrogen-bonded assembly of cytocompatible macromolecules with antioxidant and anti-inflammatory characteristics. This project is particularly timely since current islet encapsulation systems are challenging for transplantations due to high cytotoxicity and the requirement for large injection volumes. The design of novel immunoprotective materials will open new prospects for developing biomaterials with unique characteristics having applications in various bio-related areas such as bioengineering and tissue engineering. The awarded project will develop interdisciplinary collaborative research which should stimulate awareness of the needs of the UAB biomedical research community for specialized polymer-based biomaterials as novel platforms for cell transplantation therapy.
