Active Research Projects
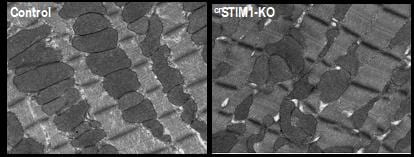
The physiological role of STIM1 in the heart: Despite improvements in the treatment of heart disease the incidence of heart failure continues to increase and mortality rates remain high. Dysregulation of Ca2+ homeostasis is a common element underlying the etiology of many cardiac diseases; however, the development of therapeutics focused on accepted Ca2+ handling pathways has been limited. This suggests that our current understanding of cardiomyocyte Ca2+ handling is incomplete. STromal Interaction Molecule-1 (STIM1) is a ubiquitous, highly conserved protein that plays a central role in regulating Ca2+ dependent transcription. In non-excitable cells, STIM1 regulates store-operated calcium entry (SOCE), where an initial release of Ca2+ from the ER, typically induced by an IP3 generating agonist, triggers an influx of Ca2+ across the plasma membrane. STIM1 regulated Ca2+ signaling is widely recognized as a core element of all mammalian Ca2+ signaling systems; however, our knowledge about the physiological role of STIM1 in adult cardiomyocytes is very limited. We have shown that cardiac-specific deletion of STIM1 leads to a dilated cardiomyopathy demonstrating that STIM1 is essential for maintenance of adult cardiomyocyte homeostasis. Our studies have also raised the possibility that STIM1 is a previously unrecognized regulator of cardiac metabolism and mitochondrial function. Work on this project is currently funded by a mulit-PI NIH grant entitled STIM1 and its role in regulating cardiac metabolism.
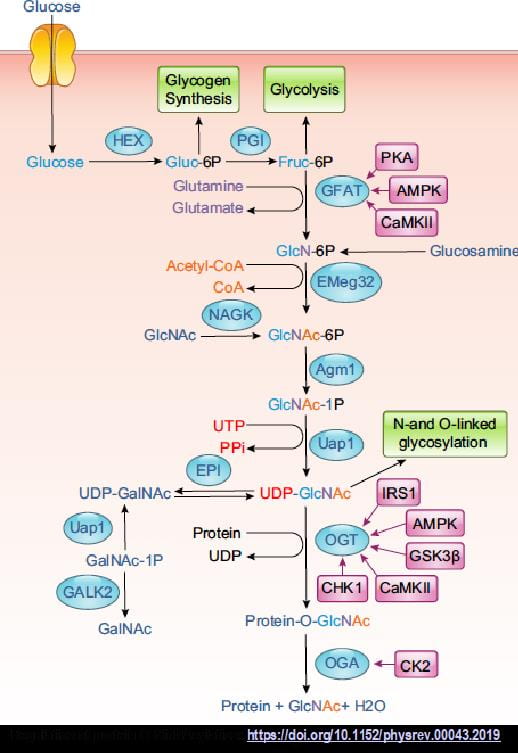
Mechanisms regulating protein O-GlcNAcylation in the heart: The post-translational modification of serine and threonine residues on proteins by O-linked N-acetyglucosamine (O-GlcNAc), is increasingly recognized as being as abundant as phosphorylation and playing a similarly important role in regulating diverse cell functions including: cell cycle, transcription, protein degradation, mitochondrial function, autophagy, circadian rhythm, and cell survival. UDP-GlcNAc, the end product of the hexosamine biosynthesis pathway (HBP), is the obligate substrate for O-GlcNAc transferase (OGT), which catalyzes the attachment of O-GlcNAc to proteins, and is subsequently removed by O-GlcNAcase (OGA). Long considered to be primarily regulated by substrate availability, there is growing evidence that short term changes in O-GlcNAc levels occur in an agonist dependent manner. Furthermore, subcellular redistribution of OGT and OGA also appears to have a significant role in O-GlcNAc regulation of cell function. Thus, nutrient-independent mechanisms also likely contribute to regulating O-GlcNAc homeostasis. Despite the association of O-GlcNAc with cardiac disease, our knowledge of the basic regulatory mechanisms in the normal healthy heart remains limited. However, there are clear indications that it contributes to the regulation of normal physiological processes in the heart. Short-term activation of O-GlcNAc levels have been shown to regulate cardiac metabolism and influence housekeeping processes such as autophagy and the cardiomyocyte circadian clock that are key to maintaining healthy cardiomyocytes. Sustained increases in cardiac O-GlcNAc levels have been associated with heart disease such as cardiac hypertrophy and diabetic complications, which has led to targeting O-GlcNAcylation as a potential therapeutic strategy. However, a lack of understanding of the fundamental biology underlying how O-GlcNAc regulates normal cardiomyocyte function, raises concerns regarding this strategy. Therefore the goal of this project is to determine the role of protein O-GlcNAcylation in contributing to cardiomyocyte resilience in response to physiological stimuli. Work on this project is currently funded by a mulit-PI NIH grant entitled The Role of Protein O-linked N-acetylglucosamine in Regulating Cardiac Physiology.
Latest Publications
Click here to view laboratory publications from 2011-2021.
