Murphy-Ullrich Lab
The goal of our lab is to understand the role of certain components of the extracellular matrix (ECM) in regulating cellular and growth factor responses to injury and repair and in cancer. Our primary focus is on elucidating the functional and pathologic significance of thrombospondin-1 (TSP-1) control of latent TGF-β activation in fibrotic disease and in the tumor microenvironment. Specific projects include 1) determining the role of cyclic ocular strain (a mimic of elevated intraocular pressure) in inducing the TSP-1/TGF-β axis in glaucoma and its role in ocular ECM remodeling; 2) determining the role of TSP-1/TGF-β pathway in the bone marrow microenvironment as it pertains to tumor immunosuppression and drug resistance in multiple myeloma; and 3) development of small molecule antagonists of the TSP-1/TGF-β pathway.
Mission
i) To delineate the mechanisms of extracellular matrix control of cell function in health and disease.
ii) To provide an intellectually rich, challenging, and safe environment for employees and trainees of all backgrounds to acquire the training and skills to become a productive member of the scientific community.
iii) To promote the importance of the study of extracellular matrix both within and beyond the immediate field.
Research Statement
The Extracellular Matrix (ECM) is the network of proteins, glycosaminoglycans, and growth factors present outside of cells. ECM enables multicellular life! ECM acts as a sensor of the cellular microenvironment and it acts as a communicator of this information, either biochemical or biomechanical, directly to cells to regulate cell signaling, differentiation, proliferation, apoptosis, shape, adhesion, and motility. ECM is made by cells and its specific composition is determined by the phenotype of the cell making the ECM. Collagens, laminins, fibronectin, heparan sulfate proteoglycans, and hyaluronan are widely expressed ECM components. ECM composition and structural organization can vary with development, cellular differentiation, and disease state. For example, the expression of certain ECM components is altered in a wound or scar and in the connective tissue (stroma) surrounding tumors. The ECM has profound effects on critical cellular processes and many diseases reflect an altered ECM, including cancer and fibrosis.

Matricellular proteins and thrombospondin-1 (TSP-1) Dr. Murphy-Ullrich has expertise in the extracellular matrix with a focus on extracellular matrix remodeling in disease. She has extensively studied the ECM protein thrombospondin-1 (TSP-1). TSP-1 is a member of a group of ECM proteins called “matricellular proteins.” In contrast to ECM proteins such as collagens, laminin, and fibronectin, matricellular proteins do not provide primary structural input into the ECM. They do interact with cellular receptors, other ECM molecules, and growth factors, however. They are thought to primarily regulate cell functions and ECM organization (see review by Murphy-Ullrich and Sage, Matrix Biology 2014, 37:1-14.) She identified the state of intermediate adhesion triggered by TSP-1 and similar matricellular proteins such as tenascin-C and SPARC and she has investigated their roles in cell-extracellular matrix adhesion, cell migration, and cell resistance to anoikis.
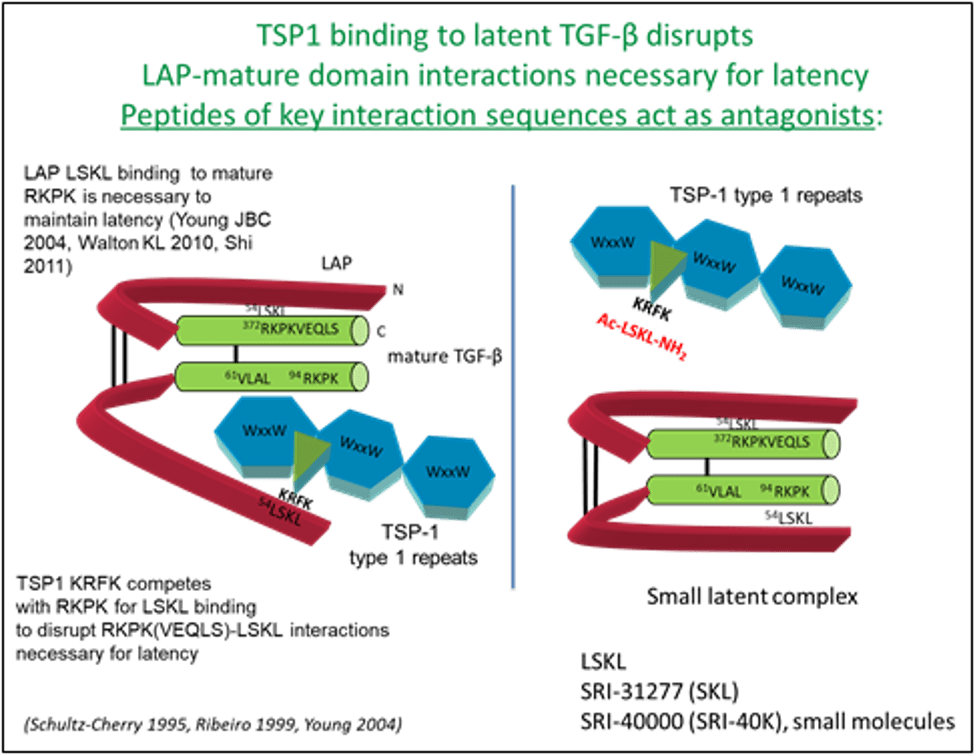
Her lab made the important discovery that TSP-1 is a regulator of latent TGF-β activation and established TSP-1 as a significant factor in regulating TGF-β activation in a number of disease processes. Her lab identified critical sites in TSP-1 that induce TGF-β activation (KRFK) and also sites in latent TGF-β necessary for latency. Based on this work, she developed tools to probe the involvement of TSP-1 in regulation of latent TGF-β in various fibrotic disease processes, including diabetic nephropathy and cardiomyopathy. The initial tool was a tetrapeptide (LSKL). Recent work in collaboration with Southern Research has been focused on identifying pre-clinical peptide and small molecule compounds based on LSKL for antagonism of the TSP-1/TGF-β pathway in fibrosis and various cancers. One such lead, SRI31277, has recently been shown to reduce myeloma tumor burden, stromal IL-6 levels, and osteolytic bone disease in pre-clinical models of aggressive systemic human myeloma, a disease of malignant plasma cells in the bone marrow with significant bone destruction. SRI31277 has also been shown to overcome drug resistance in thyroid carcinoma in vitro and in a mouse model of myeloma. A goal of Dr. Murphy-Ullrich’s research program is to identify lead compounds and bring them to clinical trials for the treatment of fibrosis, cancer, and other diseases of aberrant TGF-β activity. Initial studies suggest that one of the newly developed small molecule antagonists reduces lung fibrosis in a rat bleomycin model and immune dysfunction in myeloma. This work is reviewed in Murphy-Ullrich JE, Suto MJ. Thrombospondin-1 regulation of latent TGF-β activation: A therapeutic target for fibrotic disease. Matrix Biol. 2018 Aug;68-69:28-43. doi: 10.1016/j.matbio.2017.12.009. PMCID: PMC6015530.
The lab also investigates the role of endoplasmic reticulum (ER) chaperone and calcium binding protein calreticulin (CRT) in augmenting TGF-β signaling through NFAT, linking ER stress and fibrosis. She showed that CRT knockdown attenuates TGF-β signaling in fibrotic lung fibroblasts and in vascular smooth muscle cells and that tissue specific knockdown of CRT in the carotid artery reduces neointima formation and collagen deposition. Similarly, her lab showed that renal specific CRT knockdown attenuates renal fibrosis and albuminuria in a mouse model of diabetic nephropathy. This work establishes a molecular link between ER stress and fibrosis.
Click here to view lab publications.